-
Ultrafast Excited-State Dynamics of Donor-Acceptor Biaryls: Comparison between Pyridinium and Pyrylium Phenolates
R. Letrun, M. Koch, M.L. Dekhtyar, V.V. Kurdyukov, A.I. Tolmachev, W. Rettig and E. Vauthey
The Journal of Physical Chemistry A, 117 (49) (2013), p13112-13126


DOI:10.1021/jp409646g | unige:32101 | Abstract | Article HTML | Article PDF
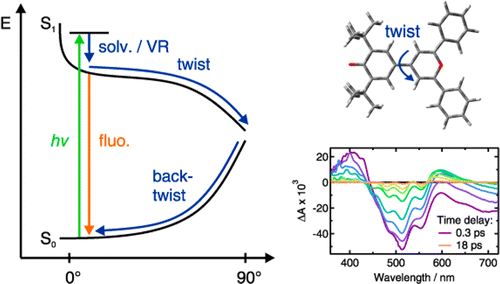
The excited-state dynamics of two donor‚Äďacceptor biaryls that differ by the strength of the acceptor, a pyridinium or a pyrylium moiety, have been investigated using a combination of steady-state solvatochromic absorption, ultrafast fluorescence, as well as visible and infrared transient absorption spectroscopies. The negative solvatochromic behavior of pyridinium phenolate indicates that the permanent electric dipole moment experiences a decrease upon S1¬†‚Üź S0¬†excitation, implying that the ground state possesses more zwitterionic character than the excited state. In contrast, pyrylium phenolate exhibits a weakly positive solvatochromic behavior corresponding to a small increase in the dipole moment upon excitation, implying more zwitterionic character in the excited than the ground state. Both compounds are therefore situated at different sides of the cyanine-limit structure, which has equally polar ground and excited states. Despite these differences, both molecules exhibit qualitatively similar excited-state properties. They are characterized by a very short fluorescence lifetime, increasing from about 1 to 20 ps, when varying solvent viscosity from 0.4 to 11 cP. There are, however, characteristic differences between the two compounds: The excited-state lifetimes of the pyrylium dye are shorter and also depend somewhat on polarity. The ensemble of spectroscopic data can be explained with a model where the emitting Franck‚ÄďCondon excited state relaxes upon twisting around the single bond between the aryl units to a point where the excited- and ground-state surfaces are very close or intersect. After internal conversion to the ground state, the distorted molecule relaxes back to its equilibrium planar configuration, again largely dependent upon solvent viscosity. However, in this case, the kinetics for the pyrylium dye are slower than for the pyridinium dye and the polar solvent-induced acceleration is significantly stronger than in the excited state. This difference of kinetic behavior between the two compounds is a direct consequence of the change of the electronic structure from anormal¬†to an¬†overcritical¬†merocyanine evidenced by steady-state spectroscopy.
